The advisory committee of the Food and Drug Administration (FDA) in the US has endorsed Pfizer’s Covid-19 vaccine for children aged between 5 to 11 years of age.
The FDA clearance came after the agency’s Vaccines and Related Biological Products Advisory Committee voted on Tuesday for a smaller dose of the Pfizer vaccine for young children.
Similarly, Centers for Disease Control and Prevention vaccine advisory group is expected to make its own recommendation next week.
If authorised, the vaccine regimen would benefit nearly 28 million more kids in the US to get protection against the virus as the delta variant spreads. Further, the move would make nearly the entire US population eligible for a Covid shot, with only children aged 4 and younger remaining.
The US government has procured enough vaccines to inoculate all 5- to 11-year-olds in the US, which means once the FDA and CDC clears authorisation, it will be immediately rolled out.
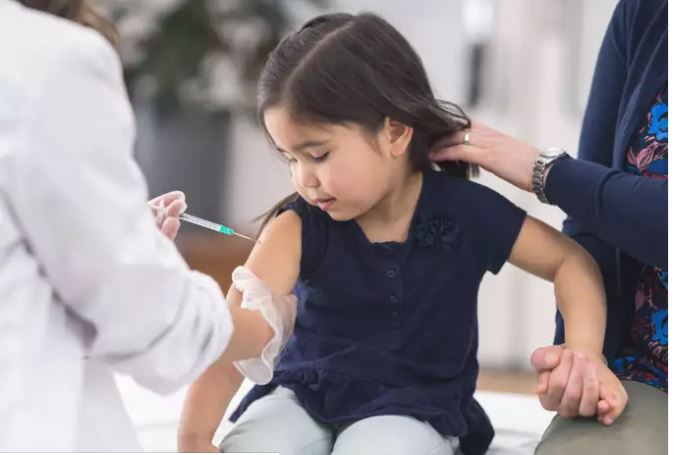
The US will distribute the shots in smaller doses and with smaller needles to make it easier for pediatricians and pharmacists to administer to kids, the report said.
Pfizer had, earlier this month, applied to the FDA for authorising its vaccine for kids ages 5 to 11. As per the data published by the company, a two-dose regimen of 10 micrograms — a third of the dosage used for teenagers and adults — is safe and generated a 90.7 per cent strong immune response in a clinical trial.



 Ms Kalinga
Ms Kalinga