CSIR Gets Green Signal From DGCI To Begin Clinical Trial Of Drugs For Covid-19 Treatment
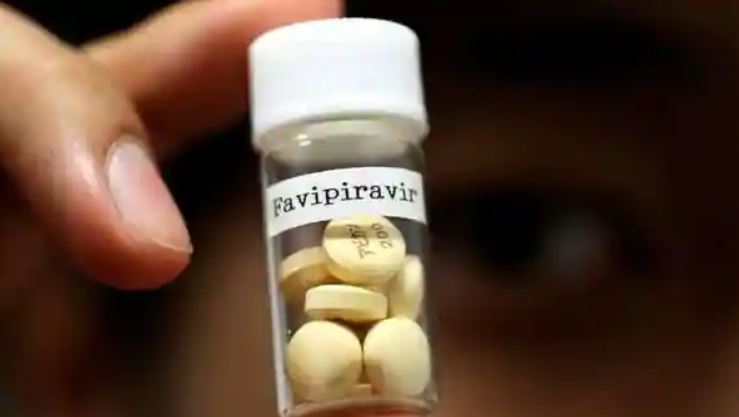
New Delhi: The Drug Controller General of India (DGCI) has given its approval to the Council for Scientific and Industrial Research (CSIR) for its two clinical trial drugs – ‘favipiravir’ and ‘phytopharmaceutical’ – to treat coronavirus patients.
CSIR Director General Shekhar Mande said that they will start the clinical trial within a week.
Favipiravir is a drug which is used to treat influenza that has a very broad spectrum of RNA polymerase. It is mostly used in Japan, China and some other countries.
The CSIR is also exploring Cocculus Hirsutus as a biological medicine or phytopharmaceutical. Cocculus Hirsutus is a native herb used by tribes in the country. It is also already being tested as medicine for dengue for its efficacy to combat COVID-19.
Phytopharmaceutical is essentially an herbal medicine extracted from plants. It is a cocktail of different compounds but has a biological origin from a plant. The Food and Drug Administration (FDA) of United States call it botanical; however, it is called phytopharmaceutical by DCGI in India.
(With inputs from dailypioneer.com)



 Ms Kalinga
Ms Kalinga